
Lasers for Medical Technology
Solutions you can count on!
Medical technology at PHOTON ENERGY
With over 25 years of experience in the field, our medical department is one of our key assets. PHOTON ENERGY offers solutions for a wide variety of medical applications including autoclavable markings on stainless steel, aluminium workpieces or implants. Our systems can easily be integrated into a UDI-compliant production.
Certified processes for the medical industry
As a certified manufacturer of medical laser systems, we are well acquainted with the special requirements of the medical industry. Consequently, we implemented the so called Good Manufacturing Practices, to ensure that our work adheres to the highest quality standards. Our medical-team will be at your side throughout every step of your project and provide you with valuable support.
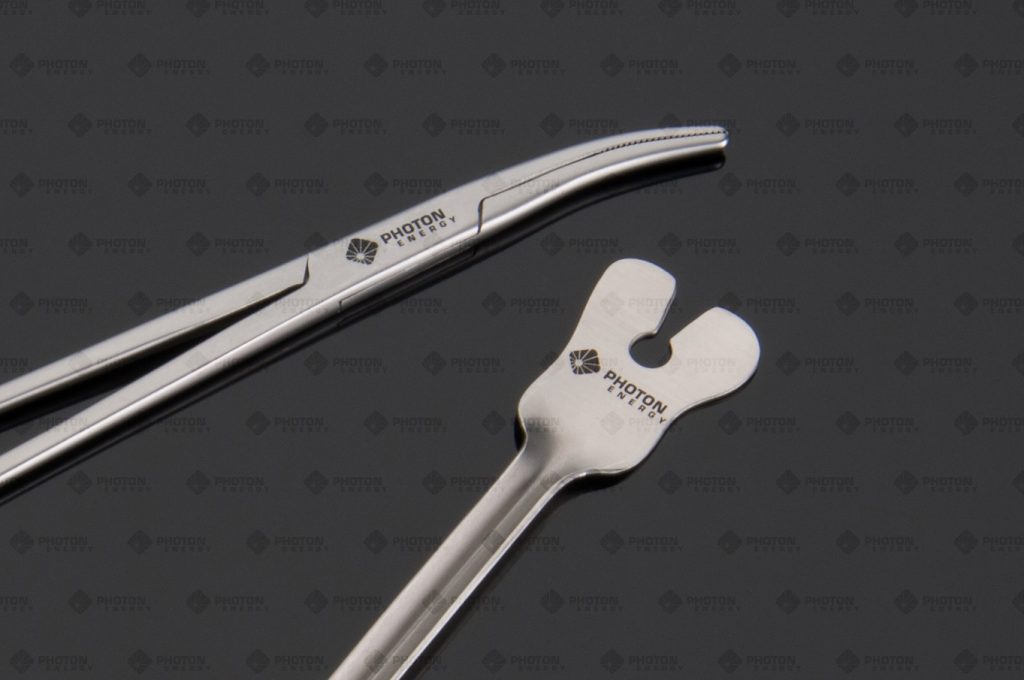
Medical Devices
PHOTON ENERGY uses its PERMAblack-technology to mark surgical instruments. Compared to the technique typically used by our competitors, our disruptive approach distinguishes itself through significant advantages.

Implants
Lasers are used in the production of various implants like hip sockets. Here as well, the marking process with lasers has many benefits.
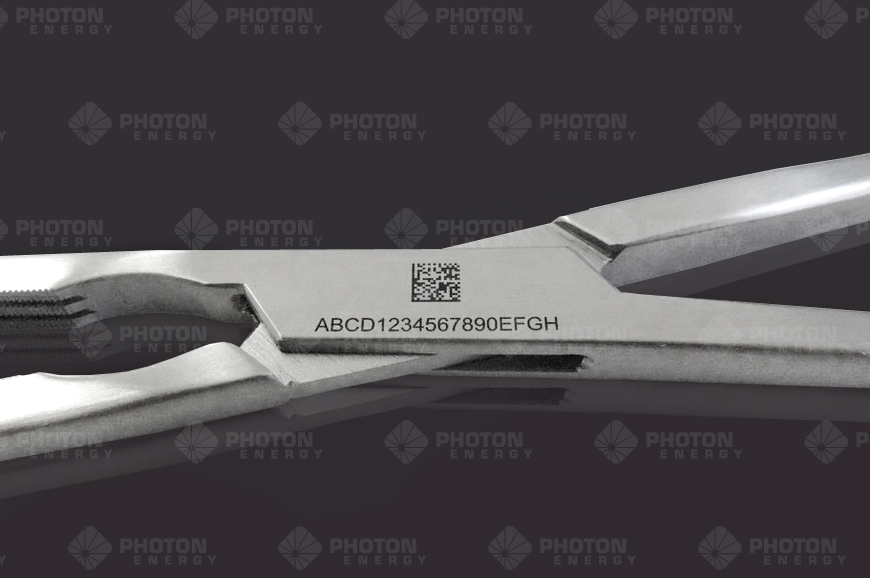
UDI-Markings
To support the implementation of UDI requirements, PHOTON ENERGY has developed a software add-on to PHOTONmark that allows to seamlessly integrate our marking systems into the UDI-process.
Good Manufacturing Practices
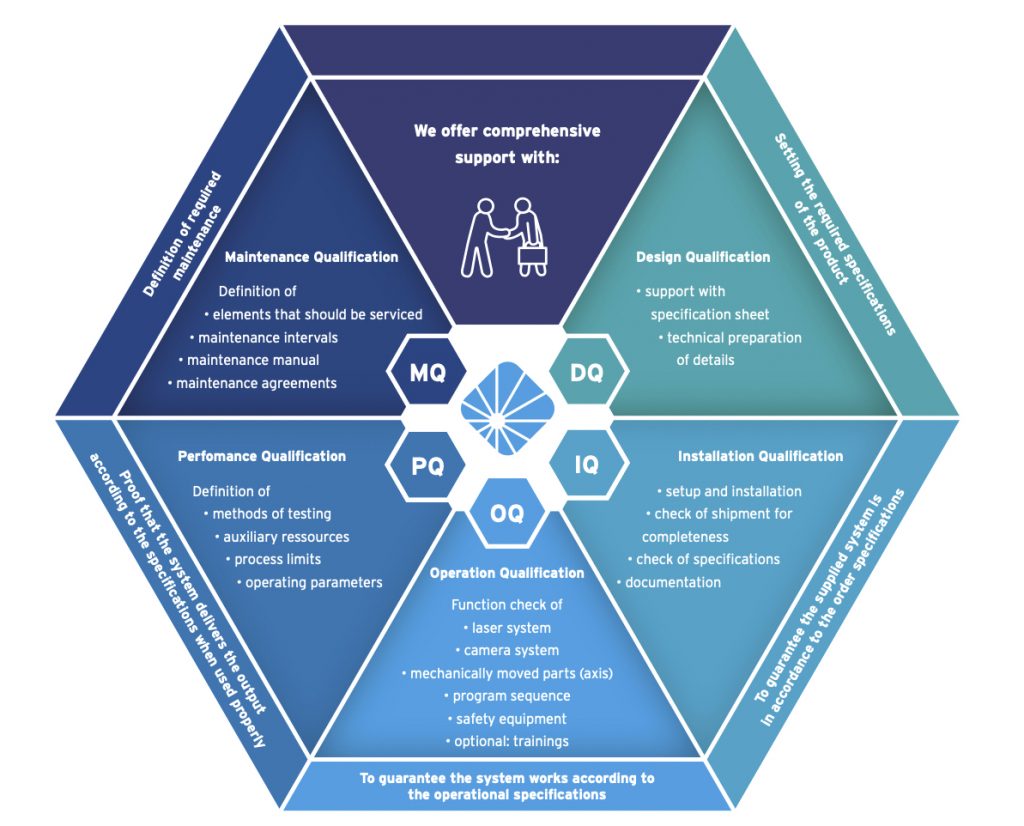
Whitepaper:
DQ, IQ, OQ, PQ, MQ
This comprehensive white paper gives an introduction to process validation and device qualification in the context of UDI and MDR regulations.



