In order to be able to track all relevant information about the product and to increase the safety of medical devices, each medical instrument has to be marked in the form of a unique, machine-readable data matrix code and with corresponding plain text.
To help you implement the UDI guidlines, PHOTON ENERGY has created a software extension that allows marking devices to be seamlessly integrated into the UDI process:
PUC – PHOTONmark UDI Control
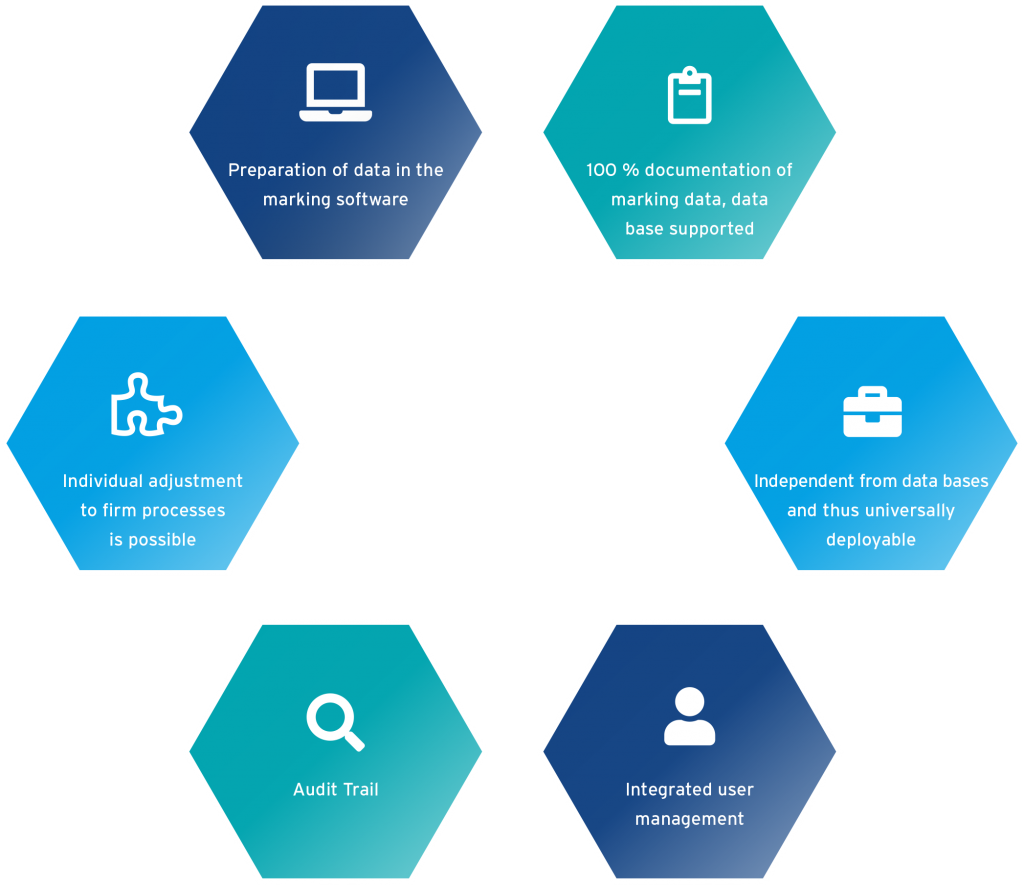
Advantages of PHOTONmark UDI Control
+ Data preparation in the marking software
+ 100% documentation of marking data, database supported
+ database independent and therefore universally applicable
+ integrated user management
+ individual adaptation to company processes possible
+ Audit trail
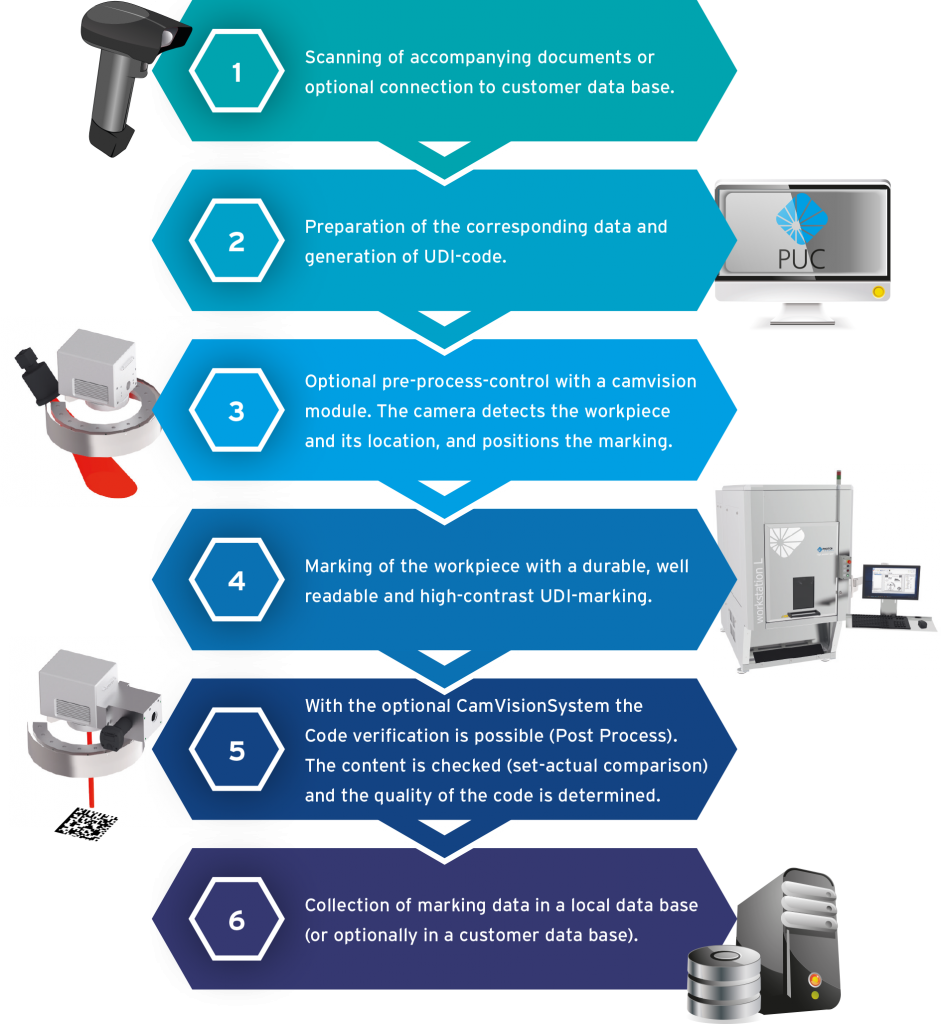
Work flow of the UDI process with PHOTONmark UDI Control
+ Scanning of lot accompanying documents
+ Preparation of data
+ Optional pre-process control
+ Labeling of the workpiece
+.Optional code verification with camera module
+ Documentation of marking data
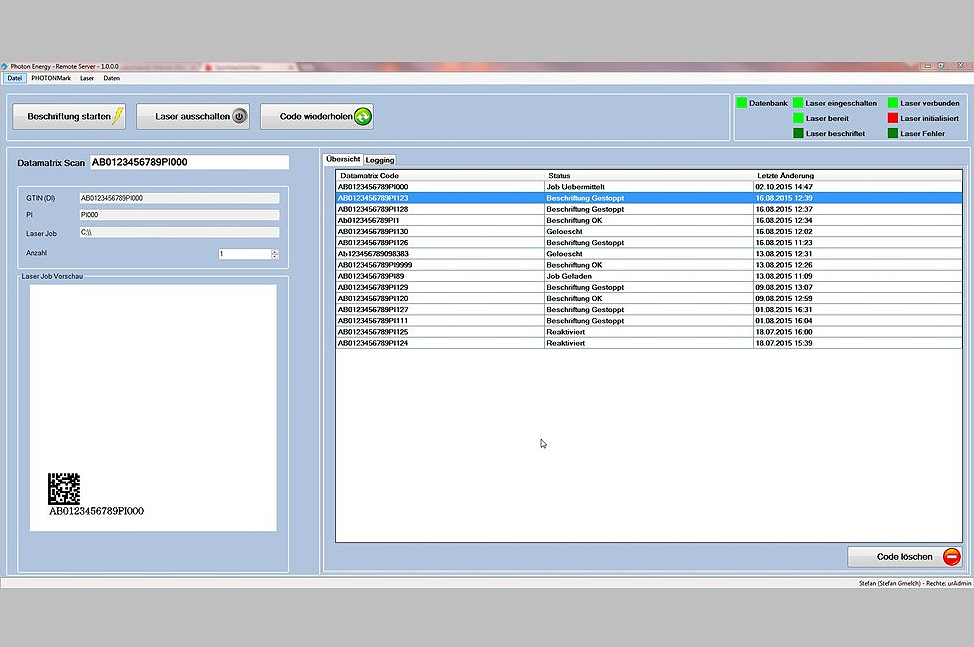
Whitepaper: Process validation and device qualification
This comprehensive white paper gives an introduction to process validation and device qualification in the context of UDI and MDR regulations.